Cost Effective Analysis of Second Line Therapies in Relapsed Refractory Multiple Myeloma in Indian Scenario
@ Indian Myeloma Congress, 2018
Suman Kumar, Army Hospital (Research & Referral), New Delhi
10 Feb 2017
Introduction
Background
Incurable malignancy of plasma cells
Most patients diagnosed above 55 years
Chronic disease with multiple remissions and relapses
Negative impact on patient's overall health related QoL
Relapsed Refractory Multiple Myeloma
Improved survival in past 20 years (5 years survival 29.2% in 1992 to 50.2% in 2012)1
It remains incurable with multiple relapses with multiple lines of treatment
Very poor prognosis with median OS of 9 months2
Recently Novel Agents approved for RRMM
- Pomalidomide (IMId)
- Daratumumab (anti CD 38 antibody)
- Carfilzomib (Proteosome Inhibitor)
- Panobinostat (HDAc inhibitor)
Major economic burden on individuals and society
References
- CAAC:CAAC21332
- After previous reference
Parent Study
Christopher G. Pelligra, Kejal Parikh, Shien Guo et al.
Cost-effectiveness of Pomalidomide, Carfilzomib, and Daratumumab for the Treatment of Patients with Heavily Pretreated Relapsed–refractory Multiple Myeloma in the United States
Clinical Therapeutics, Volume 39, Issue 10, 2017
http://www.sciencedirect.com/science/article/pii/S0149291817308998
Conduct of study
Compared three protocols
Pomalidomide-dexamethasone (POMd): MM-002 trial
Single agent Daratumumab (DARA): SIRIUS trial
Single agent Carfilzomib (CARF): PX-171-003-A1 trial
Multiple relapsed or refractory patients (Median previous therapies: 5)
Absence of head to head comparison trial
Matching adjusted indirect comparison done to get unbiased estimate of relative efficacy
Parametric fits were made for POMd arm of MM-002 data and HR were derived for DARA and CARF from their respective trials
State diagram for outcomes
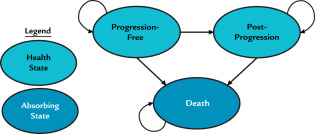
28 days cycles
Distribution of second line therapies (in %)
| Initial Line | POM-d | DARA | CAR | LEN-d | BORT | THAL-d | PanVD |
|---|---|---|---|---|---|---|---|
| POM-d | 0 | 7.5 | 38.4 | 22.2 | 17.8 | 6.6 | 7.5 |
| DARA | 40.2 | 0 | 24.8 | 14.4 | 11.5 | 4.3 | 4.8 |
| CARF | 50.2 | 6.0 | 0 | 18.0 | 14.4 | 5.4 | 6.0 |
Definitions of outcome measures
Progression free survival
Time from randomisation to disease progression (IMWG criteria) or death
Definitions of outcome measures
Progression free survival
Time from randomisation to disease progression (IMWG criteria) or death
Post progression survival
Time from disease progression till death
Definitions of outcome measures
Progression free survival
Time from randomisation to disease progression (IMWG criteria) or death
Post progression survival
Time from disease progression till death
Fatal progression probability
Per cycle probability that a patient in PF state would die
Definitions of outcome measures
Premature treatment discontinuation
Time from randomisation till discontinuation of treatment prior to disease progression due to unacceptable toxicity, patient's preference or other reasons
Definitions of outcome measures
Premature treatment discontinuation
Time from randomisation till discontinuation of treatment prior to disease progression due to unacceptable toxicity, patient's preference or other reasons
Adverse Events
4 adverse events included (Grade 3/4, occuring in > 5% of patients on treatment)
- Anemia
- Fatigue
- Neutropenia
- Thrombocytopenia
Percentages converted to monthly incidence rates
Utilities
Weights assigned to each state for calculating QALM lived
PF state: 0.7 to 0.76
PP state decrement: -0.084 to -0.025
AE decrement: -0.049 to -0.009
Present Study
Definitions and Assumptions
Outcome parameters
All outcome parameters taken from the Parent trial
Adverse event parameters were derived from the percentages given in the original trials and converted into per cycle incidence rates
Fatigue was not considered as AE
Economic parameters
All costings were done with Jan 2018, which was taken as base time
All costings were converted to per cycle unit costs
Annual inflation rate of 3.5% to 6% was assumed
Annual discount rate of 3% was used
Subclassification of costs
Regimen associated costs (= Regimen cost + Cost of adjuncts)
Cost of prophylaxis (VTE/Herpes) (= Cost of adjunct)
Cost of AEs (= PRBC txn + Plt txn + GCSF administration + 7 days of 3rd generation cephalosporin [10% of neutropenia])
Health care resource cost stratified according to health state (= Clinical Visits + CBC + Biochemistry + SPEP/SFLC/SIFE)
Regimen related costs (per cycle)
| Regimens | Variable Cost (INR), (unit) | Fixed Cost (INR) |
|---|---|---|
| POM-d | - | 20080.00 |
| DARA (cycles 1-2) | 2400.00, (per kg) | 4200.00 |
| DARA (cycles 3-6) | 1200.00, (per kg) | 2100.00 |
| DARA (cycles > 6) | 600.00, (per kg) | 1050.00 |
| CARF (cycle 1) | 24675.30, (per sq m) | - |
| CARF (cycles 2-12) | 26973.00, (per sq m) | - |
| CARF (cycles > 12) | 17982.00 (per sq m) | - |
| LEN-d | - | 15788.00 |
| BORT | - | 45200.00 |
| THAL-d | - | 7568.00 |
| PAN-V-d (cycles 1-8) | - | 110280.00 |
| PAN-V-d (cycles > 8) | - | 87640.00 |
Regimen related costs (per cycle)
Cost of Prophylaxis
VTE: (90% on Aspirin and rest on Enoxaparin) INR 1459.20
Antiviral: INR 360.00
Cost of AEs (per episode)
Anemia
- 2 units of PRBC transfusion
- INR 2100.00
Neutropenia
- 5 days of GCSF transfusion and 7 days of 3rd generation cephalosporin (10% of neutropenia)
- INR 5000.00
Thrombocytopenia
- 1 unit of SDP/6 units of PRP
- INR 1800.00
Health care resource utilisation cost (per cycle)
| Headings | Unit Cost (INR) | PFS (T+) | PFS (T-) | PPS |
|---|---|---|---|---|
| Hematologist clinical visit | 1000.00 | 2000.00 | 1000.00 | 3000.00 |
| Full Blood Count | 350.00 | 700.00 | 350.00 | 1050.00 |
| Biochemistry (RFT/LFT) | 1400.00 | 2800.00 | 1400.00 | 4200.00 |
| SPEP | 700.00 | 233.33 | 116.67 | 233.33 |
| SIFE | 5000.00 | 1666.67 | 833.33 | 1666.67 |
| SFLC | 6500.00 | 2166.67 | 1083.33 | 2166.67 |
| Total | - | 9566.67 | 4783.33 | 12316.67 |
Conduct of Study
(Computer simulation study)
Sample Size
Horizon of 3 years was chosen for the simulation (stable economic condition). Patients were censored after 36 months.
100 patients for each of the 3 arms
Total of 500 simulations were done
Few examples (POM-d arm)
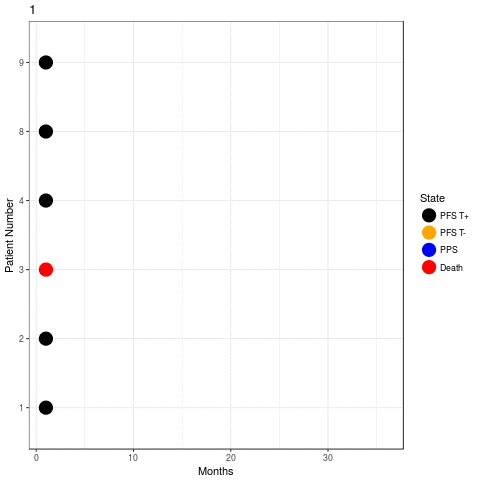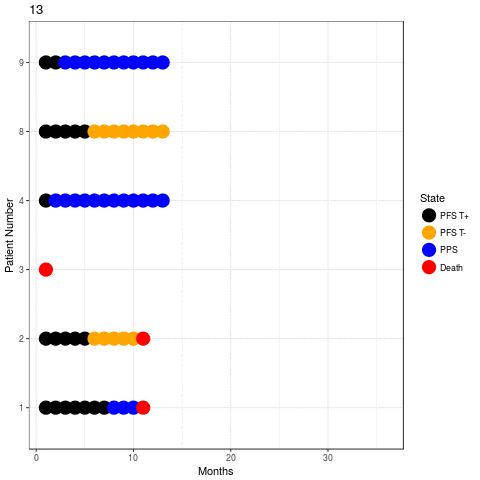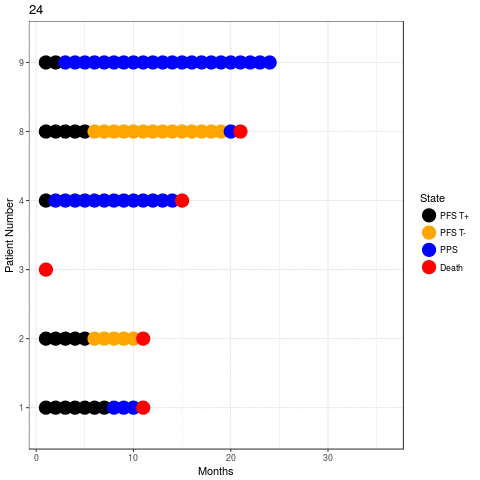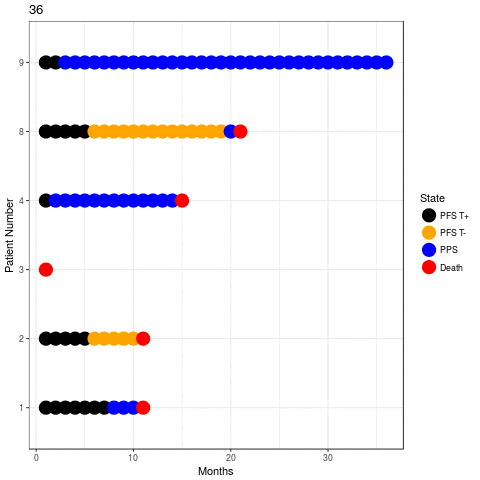
Results
Clinical Outcomes
Median Overall Survival (months)
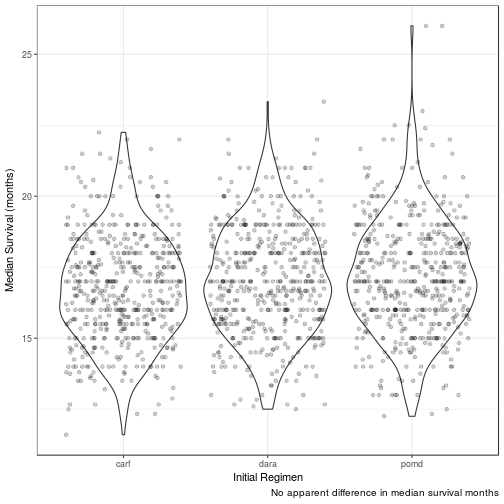
| init_reg | mean_median_surv | 95LCL | 95UCL | 25centile | 75centile |
|---|---|---|---|---|---|
| pomd | 17.1570 | 13.3333 | 21.3575 | 15.8512 | 18.4071 |
| dara | 17.0114 | 13.5000 | 21.0000 | 15.6667 | 18.3333 |
| carf | 16.7497 | 13.5000 | 20.6667 | 15.5000 | 18.0000 |
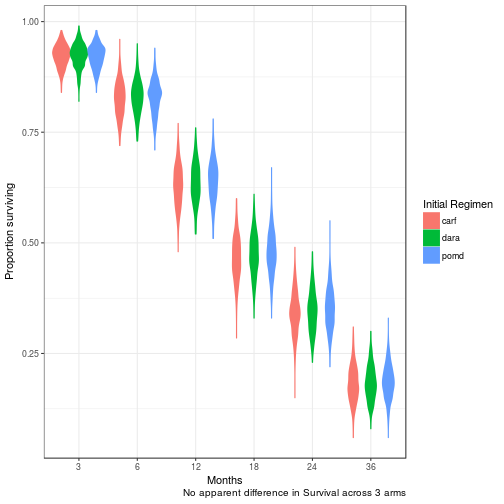
Overall Survival at 3, 6, 12, 18, 24, 36 months
Median Progression Free Survival (months)
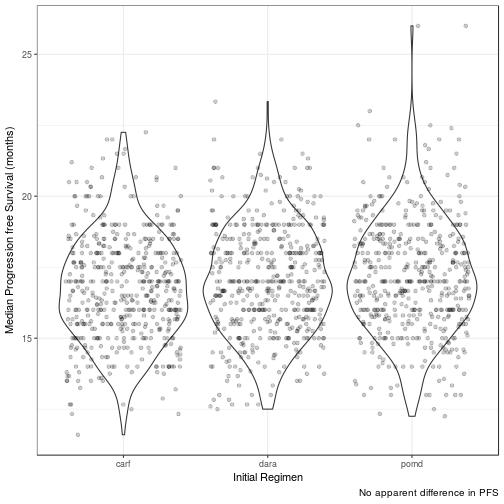
| init_reg | mean_median_pfs | 95LCL | 95UCL | 25centile | 75centile |
|---|---|---|---|---|---|
| pomd | 17.1425 | 13.3333 | 21.3575 | 15.8000 | 18.4000 |
| dara | 17.0007 | 13.5000 | 20.8812 | 15.6667 | 18.2708 |
| carf | 16.7488 | 13.5000 | 20.6667 | 15.5000 | 18.0000 |
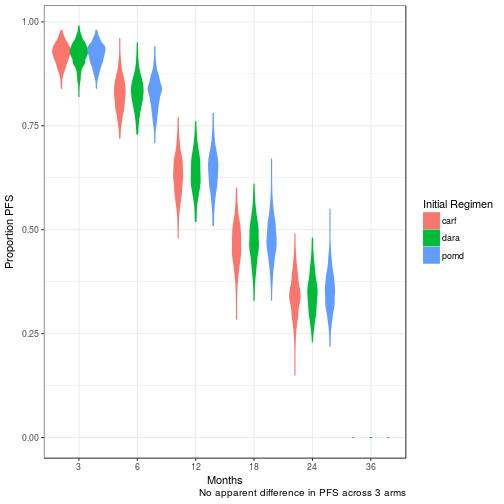
PFS at 3, 6, 12, 18, 24, 36months
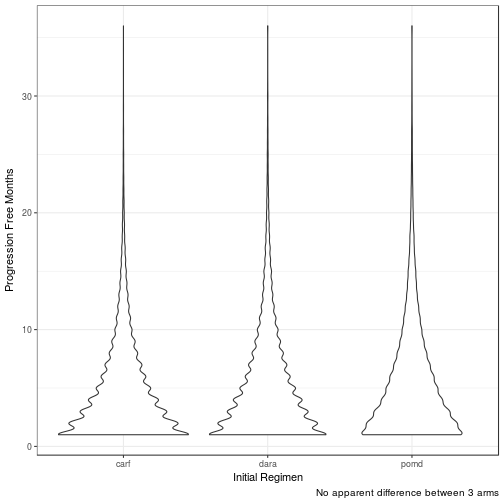
Progression Free Life Months (PFLM)
| init_reg | med_pfm | 95LCL | 95UCL | 25tile | 75tile |
|---|---|---|---|---|---|
| carf | 4 | 3 | 4 | 3 | 4.0 |
| dara | 4 | 3 | 5 | 4 | 4.0 |
| pomd | 4 | 3 | 5 | 4 | 4.5 |
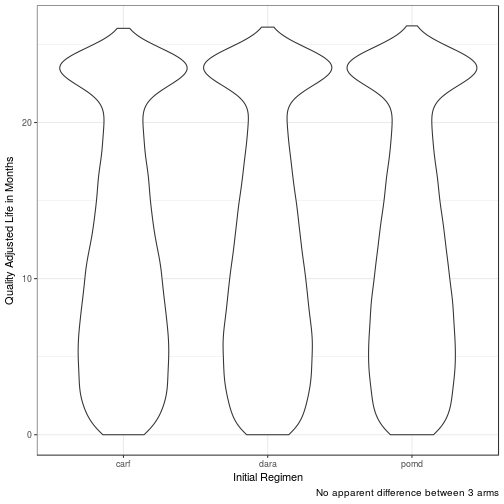
Quality Adjusted Life Months (QALM)
| init_reg | med_qalm | 95LCL | 95UCL | 25tile | 75tile |
|---|---|---|---|---|---|
| carf | 11.0293 | 8.8127 | 13.7548 | 10.2393 | 11.9131 |
| dara | 11.2297 | 9.0112 | 13.7750 | 10.3453 | 12.1640 |
| pomd | 11.3767 | 8.9134 | 14.0928 | 10.5132 | 12.2253 |
No difference in clinical efficacy between all three arms
Economic Outcomes
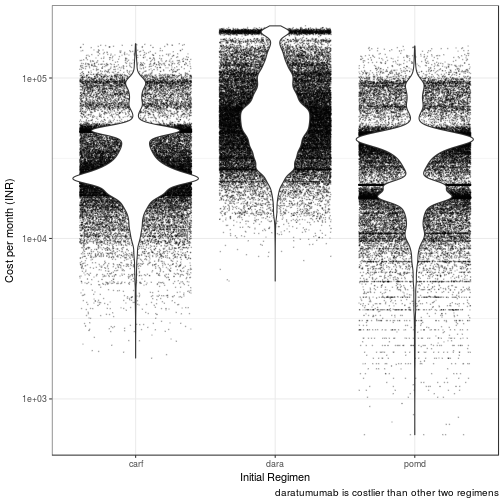
Regimen associated Discounted Cost
| init_reg | med_cost_month | 95LCL | 95UCL | 25tile | 75tile |
|---|---|---|---|---|---|
| dara | 55143.72 | 48638.94 | 63355.32 | 52726.57 | 57723.10 |
| pomd | 32370.84 | 21656.73 | 37982.45 | 29379.04 | 34890.15 |
| carf | 25769.79 | 23650.16 | 29015.03 | 24905.19 | 26911.36 |
Cost per QALM
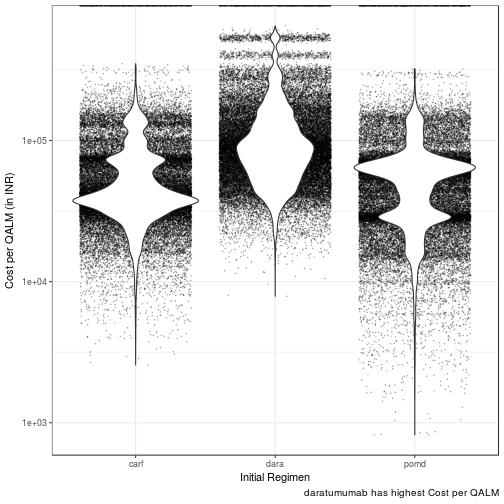
| init_reg | med_cost_qalm | 95LCL | 95UCL | 25tile | 75tile |
|---|---|---|---|---|---|
| dara | 86609.35 | 76026.52 | 99518.78 | 82721.20 | 90635.68 |
| pomd | 55897.85 | 44735.16 | 61518.81 | 51986.42 | 58222.93 |
| carf | 41495.18 | 37974.16 | 47988.91 | 40129.71 | 43575.42 |
Conclusions
All the three treatment arms are equally effective clinically
Daratumumab has the highest median cost per QALM lived of INR 86609.00 (95% CI: 76027.00 - 99519.00). Incremental cost per QALM lived is INR 30711.00 than POM-d
Carfilzomib has the lowest median cost per QALM lived of INR 41495.00 (95% CI: 37974.00 - 47989.00). Incremental cost per QALM lived is INR (- 14403.00) than POM-d
Pomalidomide dexamethasone has the median cost per QALM lived of INR 55897.85 (95% CI: 44735.16 - 61518.81)